TL;DR
Risks of Contaminants in Dietary Supplements
- Widespread Use: Over two-thirds of Americans take dietary supplements, with 84% confident in their safety and effectiveness.
- Hidden Dangers: Many supplements contain heavy metals, which are linked to serious health problems like cancer, dementia, and brittle bones.
- Consumer Awareness: It’s essential for consumers to choose supplements that have been independently tested by reputable labs to ensure safety and avoid harmful contaminants.
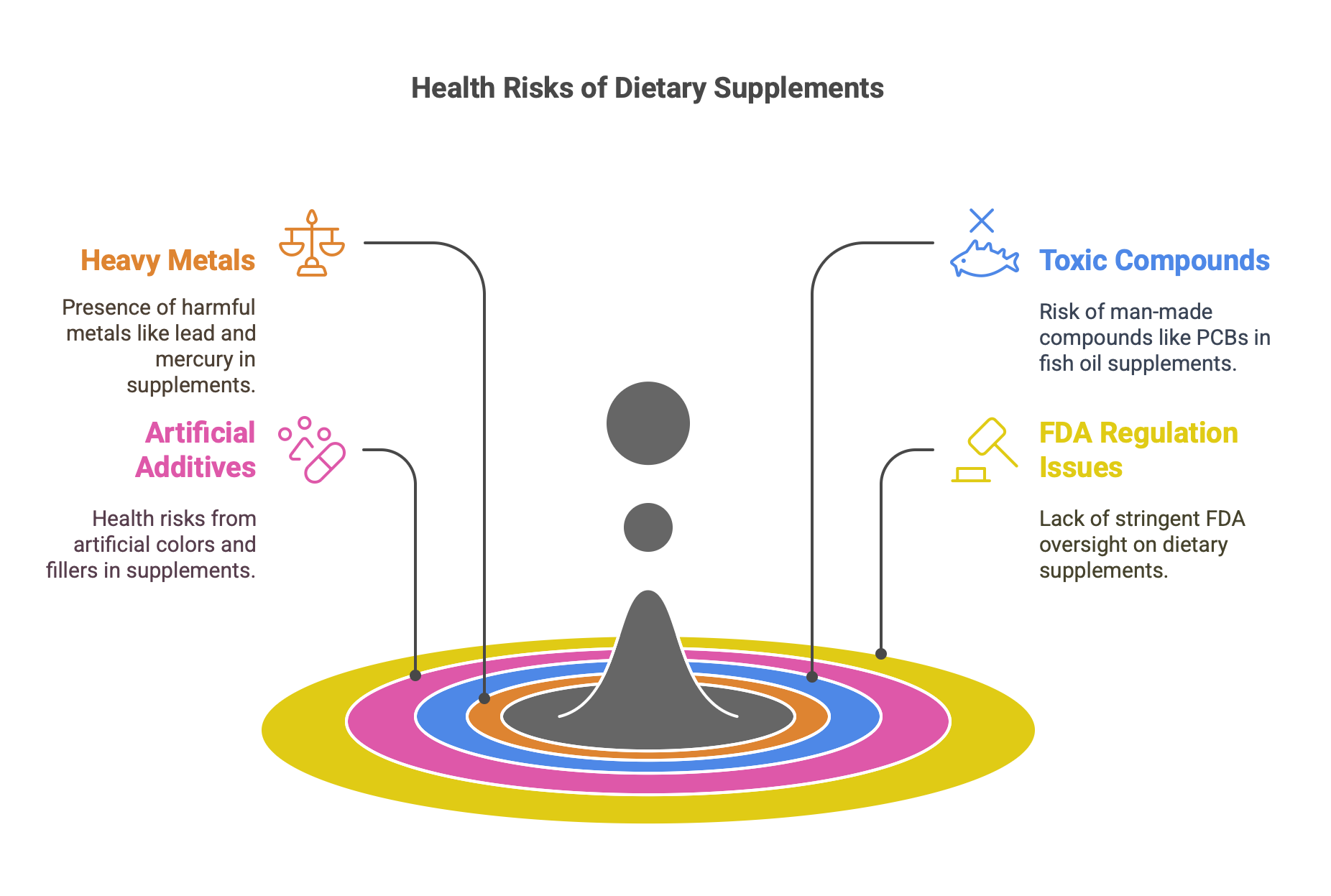
Dangerous Chemicals in Dietary Supplements
- Widespread Use and Risks: Over two-thirds of Americans take dietary supplements, with 84% believing in their safety. However, many contain harmful chemicals.
- Heavy Metals: Supplements may have dangerous levels of lead, mercury, cadmium, and arsenic, linked to cancer, dementia, and brittle bones.
- Toxic Compounds: Fish oil supplements often contain PCBs, harmful man-made chemicals causing illness.
- Artificial Additives: Coloring agents, fillers, and binders, used to enhance appearance and taste, can harm health.
- FDA Measures: The FDA’s Dietary Supplement Ingredient Advisory List flags potentially unsafe ingredients to protect consumers.
Common Contaminants in Vitamins and Supplements
- Health Risks: Studies show 5% of supplements exceed arsenic limits, 2% contain excessive lead and cadmium, and 1% have unsafe mercury levels.
- FDA Intervention: In 2019, the FDA seized 300,000 supplement bottles due to high lead content.
- Consumer Trust: Despite 84% of Americans believing in supplement safety, products without independent verification may harbor serious risks.
FDA Approval Process and Consumer Safety in Dietary Supplements
- NDI Notification: The Federal Food, Drug, and Cosmetic Act requires manufacturers to notify the FDA about “new dietary ingredients” (NDIs) in supplements, ensuring they are safe for use.
- Regulatory Framework: While pre-approval isn’t mandatory, an NDIN is required for supplements containing ingredients not marketed in the U.S. before October 15, 1994.
- Guidance Updates: In March 2024, the FDA issued updated guidance to streamline NDIN procedures and provide clarity for manufacturers.
- Consumer Safety: Under the Dietary Supplement Health and Education Act (DSHEA), manufacturers must evaluate product safety and labeling to prevent misbranding and adulteration.
- FDA Oversight: The FDA’s Office of Food Chemical Safety ensures thorough evaluation of supplement ingredients, protecting consumers from harmful additives and misrepresented products.
Identifying Dangerous Chemicals on Supplement Labels
- Red Flags: Watch for harmful additives like red dye No. 3, titanium dioxide, brominated vegetable oil, propylparaben, and potassium bromate, often used to enhance supplement appearance, taste, or shelf life.
- Prevalence of Issues: Research by ConsumerLab.com found problems in over 20% of supplements reviewed, including the presence of prohibited or questionable substances.
- Certification: Look for NSF Certified for Sport labels, which ensure products have been independently tested for safety and quality.
- Label Awareness: Carefully examine ingredient lists to identify fillers, additives, and synthetic components that could pose health risks.
Long-term Health Effects of Contaminants in Supplements
- Heavy Metals: Chronic exposure to heavy metals, such as lead and mercury, in supplements is linked to cardiovascular diseases through vascular aging. A NHANES study highlighted their role as environmental risk factors contributing to long-term health issues.
- PCBs (Polychlorinated Biphenyls): These toxic compounds, often found in fish oil supplements, can cause liver damage, skin conditions, respiratory issues, and systemic toxicity. Their persistence and bioaccumulation amplify health risks, even at low-level exposures.
- Testing and Regulation:
- PCB levels can be monitored via adipose tissue or serum analysis, though better diagnostic tools are needed to correlate exposure with health outcomes.
- Effective monitoring and stringent regulations are critical to prevent long-term adverse effects from contaminants in dietary supplements.
Introduction
More than two-thirds of Americans take dietary supplements, with the vast majority—84%—confident in the safety and effectiveness of these products[5]. However, this confidence may be misplaced. Consumers face significant risks when using dietary supplements that have not been independently verified by reputable outside laboratories. Heavy metals, known to cause severe health issues such as cancer, dementia, and brittle bones, contaminate many of these supplements[5]. As highlighted in a new article in the Annals of Pharmacotherapy, it is critical for consumers to be aware of the potential dangers posed by contaminants in dietary supplements and to seek products that have undergone thorough testing[5].
ALVA Favourites
When you make a purchase through retailer links on our site, we may earn commission through affiliate programs. All affiliate fees ALVA receives support our mission. Learn more here
Are your products safe?
Scan your products now with the ALVA app to get a personalised risk analysis.
Dangerous Chemicals in Dietary Supplements
Dietary supplements, while popular among many consumers seeking to improve their
health, can pose significant health risks due to the inclusion of dangerous chemicals
and additives. Despite the fact that more than two-thirds of Americans take dietary
supplements and a substantial majority—84%—believe these products are safe and
effective, this confidence may be misplaced[4].
One of the primary concerns is the presence of heavy metals in supplements. Studies
have shown that a percentage of dietary supplements contain harmful levels of
metals such as lead, mercury, cadmium, and arsenic. These metals are known to
cause severe health issues, including cancer, dementia, and brittle bones. In a study
examining 121 products, 5% exceeded the safe daily consumption limit for arsenic,
while 2% had excess levels of lead, cadmium, and aluminum, and 1% contained too
much mercury[4].
Another significant concern is the use of toxic man-made compounds such as PCBs
(polychlorinated biphenyls), particularly in fish oil supplements. These compounds
can cause illness in humans, and it is crucial for consumers to select brands that
rigorously test their products for these contaminants[2].
Artificial coloring and other additives used as flow agents, binders, and fillers also
present health risks. These substances are used to improve the appearance, taste,
and manufacturability of supplements, but many have been shown to damage health.
The lack of regulation by the FDA further exacerbates this issue, as the agency has
cleared over 3,000 additives for use, many of which are detrimental to health[1].
To address these concerns, the FDA has initiated the Dietary Supplement Ingredient
Advisory List, an online tool to alert the public to chemical compounds that may not
meet legal requirements for use in supplements. This list helps flag potentially unsafe
ingredients before a final determination is made, offering some level of consumer
protection[3].
Common Contaminants in Vitamins and Supplements
In today’s health-conscious world, dietary supplements have become a staple in
many people’s routines. From vitamins and minerals to protein powders and herbal
extracts, these products promise to enhance our well-being. However, not all sup-
plements are created equal, and some may contain contaminants that pose serious
health risks[6][9].
One of the most concerning types of contaminants found in dietary supplements
are heavy metals. Heavy metals such as lead, mercury, arsenic, and cadmium
are naturally occurring elements that can be harmful when ingested in significant
amounts[6]. These metals can infiltrate supplements at various stages of production,
including through contaminated raw materials, improper manufacturing processes, or
even during packaging[6]. The health risks associated with these contaminants are
significant, with heavy metals known to cause cancer, dementia, and brittle bones[7].
Studies have shown that a notable percentage of dietary supplements contain unsafe
levels of these toxic substances. For instance, one study examining 121 products
found that 5% surpassed the safe daily consumption limit for arsenic, while 2% had
excess levels of lead, cadmium, and aluminum. Additionally, 1% of the supplements
tested had too much mercury[8]. The risks are not merely hypothetical; in June 2019,
the Food and Drug Administration seized 300,000 dietary supplement bottles due to
excessive lead levels[8].
Consumers often trust that dietary supplements are safe and effective, with 84%
of Americans confident in the safety of these products[7][8]. However, the real
risks associated with supplements not independently verified by reputable outside
labs underscore the need for vigilance and informed choices when selecting these
products[7]. Understanding the potential contaminants and prioritizing purity and
safety is critical for protecting one’s health[6].
FDA Approval Process and Consumer Safety
The Federal Food, Drug, and Cosmetic Act (FD&C Act) mandates that manufacturers
and distributors who intend to market dietary supplements containing “new dietary
ingredients” (NDIs) must notify the Food and Drug Administration (FDA) about these
ingredients[10][12]. This notification process is essential to ensure that any new
dietary ingredient in a supplement is reasonably expected to be safe under the
conditions of use recommended or suggested in the labeling[10].
The process begins with the submission of a New Dietary Ingredient Notification
(NDIN) to the FDA. The notification must include information that serves as the basis
for the manufacturer’s conclusion regarding the safety of the dietary supplement
containing the new ingredient[12]. Detailed guidance on how to submit notifications,
including timeframes, format, and method of submission, is provided by the FDA[10].
On March 5, 2024, the FDA issued final guidance titled “Dietary Supplements: New
Dietary Ingredient Notification Procedures and Timeframes” to aid manufacturers and
distributors in navigating the notification process[13]. This guidance finalizes Section
V of the 2016 draft guidance and addresses industry comments by separating the
finalized sections for ease of use[13].
It is important to note that while the FDA does not require pre-approval for dietary
supplements, the submission of an NDIN is obligatory if the supplement contains an
ingredient not marketed in the United States before October 15, 1994[15]. This step
ensures that as the dietary supplement industry grows and innovates, the safety of
new ingredients is thoroughly evaluated before they reach consumers[15].
Ensuring Consumer Safety for Dietary Supplement Ingredients
The Food and Drug Administration (FDA) oversees the regulation of dietary supple-
ments through the Human Foods Program’s Office of Food Chemical Safety, Dietary
Supplements, and Innovation[16]. Unlike conventional foods and drug products,
dietary supplements are regulated under a distinct set of regulations established by
the Dietary Supplement Health and Education Act of 1994 (DSHEA)[16]. According
to DSHEA, manufacturers and distributors are prohibited from marketing adulterated
or misbranded products. They bear the responsibility for evaluating the safety and
labeling of their products before they reach the market to ensure compliance with
the Federal Food, Drug, and Cosmetic Act, as amended by DSHEA, and other FDA
regulations[16].
The FDA’s regulatory framework mandates that companies assess the safety of di-
etary supplements and ingredients to protect consumer health. This process includes
rigorous scrutiny to prevent the inclusion of harmful additives and ensure that all
product labels provide accurate information about ingredients and their safety[16].
Through these measures, the FDA aims to safeguard consumers from potential
health risks posed by dangerous chemicals in vitamins and supplements[16].
Identifying Potentially Dangerous Chemicals on Supplement Labels
Understanding the information presented on supplement labels is crucial for ensuring
that the products you consume are safe and effective. Not all ingredients are created
equal, and some should be approached with caution or avoided altogether[17]. Many
products on the market are loaded with fillers, additives, and low-quality synthetic
ingredients that are not only ineffective but can also be harmful to your health[18].
One of the major red flags to look out for is the presence of toxic chemicals and
substances that are commonly used in supplements to enhance their appearance,
taste, smell, and shelf life[18]. For instance, chemicals such as red dye No. 3, titanium
dioxide, brominated vegetable oil, propylparaben, and potassium bromate are often
used in processed foods and can also be found in dietary supplements[17].
Based on extensive research and testing by ConsumerLab.com, which has reviewed
the labels of more than 6,000 dietary supplements, problems were found in over 20%
of them. This highlights the importance of being vigilant and recognizing potential
risks when choosing supplements[19]. Some of the top red flags include the presence
of prohibited substances on labels or in advertising, which can be indicative of a
higher risk supplement[20].
To mitigate these risks, it is recommended to choose supplements that are NSF
Certified for Sport, which indicates that the product has been tested for safety and
quality[20]. Additionally, paying close attention to the ingredient list on nutrition labels
can provide valuable insights into a product’s benefits and drawbacks, helping you
make more informed choices for your health[21].
Long-term Health Effects of Contaminants
Long-term Health Effects of Heavy Metals
Prolonged exposure to heavy metals in vitamins and supplements has been identified
as a significant health risk due to its association with cardiovascular diseases (CVD).
A large cross-sectional study published in the Journal of Translational Medicine ex-
amined data from 3,772 participants in the National Health and Nutrition Examination
Survey (NHANES) spanning from 2005 to 2016[25]. The study specifically analyzed
urinary concentrations of nine heavy metals and assessed their associations with
vascular age through estimated pulse wave velocity (ePWV) and heart vascular
age (HVA)[25]. The results indicated that heavy metal exposure is an emerging
environmental risk factor linked to CVD through its effects on vascular aging, thereby
highlighting the critical need for monitoring and regulating heavy metal levels in
dietary supplements to prevent long-term health effects[25].
Prolonged Exposure to PCBs Health Risks
Prolonged exposure to polychlorinated biphenyls (PCBs) in vitamins and supple-
ments poses significant health risks. Occupational exposure to PCBs, particularly in
individuals working in manufacturing plants that utilized these chemicals extensively,
has been linked to several adverse health effects, including increased levels of
liver enzymes, potential hepatic damage, chloracne and related dermal lesions,
and respiratory problems[22]. These findings highlight the severe health impacts
associated with relatively high levels of PCB exposure.
The public health concerns surrounding PCBs are amplified by their persistence and
bioaccumulation in the environment and human tissues. The specific health effects
of PCB exposure are influenced by the dose, duration, and the route of exposure, as
well as individual traits and habits[23]. Although low-level environmental exposure to
PCBs presents a more complex scenario requiring further validation, it is clear that
any level of exposure can potentially lead to significant health risks[22].
In animal studies, exposure to commercial PCBs has been shown to cause a
wide range of toxic responses, including acute lethality and body weight loss[22].
These effects underscore the importance of monitoring and regulating PCB levels in
consumer products, including vitamins and supplements, to mitigate long-term health
risks.
Measuring long-term exposure to PCBs can be achieved through various methods.
Due to the lipophilic nature of PCBs, they tend to accumulate in fat tissues, making
biopsied adipose tissue analysis a reliable method for assessing exposure. Serum
PCB analysis offers a less invasive alternative and can be performed by most
commercial reference laboratories. However, while these tests are useful for gauging
exposure, they may not always correlate directly with adverse health effects[24].
Therefore, ongoing research and improved diagnostic techniques are essential for
accurately assessing and addressing the health risks posed by PCBs.
FDA Measures and Strategies for Detection and Prevention
The FDA’s Human Foods Program (HFP) plays a pivotal role in ensuring that food and
dietary supplements are safe for consumption. The Office of Food Chemical Safety,
Dietary Supplements, and Innovation, commonly known as the Chemical Safety
Office, is integral to this mission. This office is tasked with ensuring that exposure
to chemicals in food is safe by employing a risk-based approach to food chemical
safety and dietary supplement policy[26]. The FDA oversees both finished dietary
supplement products and dietary ingredients, ensuring that manufacturers and dis-
tributors do not market products that are adulterated or misbranded, as mandated by
the Dietary Supplement Health and Education Act of 1994 (DSHEA)[27].
The FDA also provides guidance documents to the food industry, which reflect
the agency’s current thinking on various topics. While these documents are not
binding, they offer alternative approaches that can satisfy statutory and regulatory
requirements[28]. The rapid growth of the dietary supplement industry, spurred by
increased consumer awareness and demand for health and wellness products, has
heightened the need for stringent compliance with FDA regulations[29].
Despite these measures, there are significant gaps in the regulatory framework.
The FDA does not review or test the safety of dietary supplements before they
are marketed, leaving the agency unaware of what products are available and their
contents. Furthermore, the FDA cannot mandate recalls for supplements that may
contain illegal pharmaceuticals, posing a risk to consumers[30]. Under the DSHEA,
established supplement ingredients can be marketed without evidence of efficacy or
safety, and enforcement of new ingredient safety has been lacking[31].
To address these challenges, the FDA has the authority to stop companies from sell-
ing toxic or unsanitary dietary supplements[32]. However, stronger federal oversight
is necessary to ensure the safety of these products, as consumers increasingly take
responsibility for their own health and wellness by using dietary supplements[30][32].
Role of Third-party Certifications
Ensuring the quality and safety of dietary supplements is paramount, given that these
products are not subject to pre-market approval by the Food and Drug Administration
(FDA)[34][37]. Third-party certifications play a crucial role in helping consumers
identify safe and reliable supplements amidst a market flooded with options that may
contain undisclosed ingredients or contaminants[33][35].
Third-party supplements are those that have been tested and verified by independent
organizations, separate from the manufacturer[33]. These certifications provide an
additional layer of quality assurance, offering consumers confidence that the products
they are purchasing have undergone rigorous testing for safety, purity, and efficacy[-
35]. This is particularly important in an industry where mislabeled and contaminated
products are not uncommon, posing risks such as adverse health effects, positive
drug tests, and other negative consequences[34].
One of the most recognized certification programs is NSF/ANSI 173, which sets
stringent standards for dietary supplement products[36]. Certification programs like
NSF/ANSI 173 ensure that supplements meet established safety and quality criteria,
thus reducing the risk of contaminants and adulterants that could harm consumers-
[36].
For athletes, third-party testing is especially critical, as the presence of banned
substances in supplements can lead to serious repercussions, including disquali-
fication from competitions[37]. Organizations such as Informed Sport and NSF are
considered the gold standard in third-party testing, ensuring that sport supplements
meet the highest quality standards and are free of prohibited substances[39].
Consumers can verify the credibility of third-party certifications by looking for seals
from reputable organizations on supplement labels. These seals not only confirm
ingredient purity and compliance with current good manufacturing practices (cGMP)
but also provide assurance that the products have been independently evaluated
for quality[39]. Understanding these certifications helps consumers make informed
decisions and choose products that are both safe and effective[38].
References
[1]: 17 Harmful Ingredients to Avoid in Supplements – DailyNutra
[2]: What’s in Your Supplements? Ingredients to Avoid Vitamins – Robinson MD
[3]: FDA Unveils List of Suspected Unlawful Ingredients in Dietary Supplements
[4]: Natural supplements can be dangerously contaminated, or not even have …
[5]: Analysis: Some natural supplements can be dangerously contaminated – PBS
[6]: The Hidden Dangers of Heavy Metals and Toxins in Supplements
[7]: Analysis: Some natural supplements can be dangerously contaminated – PBS
[8]: Natural supplements can be dangerously contaminated, or not even have …
[9]: The Hidden Dangers in Your Supplements: A Deep Dive into Contaminants
[10]: New Dietary Ingredient (NDI) Notification Process | FDA
[11]: Guidance for Industry: New Dietary Ingredient Notification Procedures …
[12]: New Dietary Ingredients in Dietary Supplements – Background for …
[13]: FDA Releases Final Guidance on New Dietary Ingredient Notification …
[14]: Navigating FDA Regulations for Dietary Supplements
[15]: New Dietary Ingredients (NDIs): Exceptions & Additional … – Element
[16]: Dietary Supplements | FDA – U.S. Food and Drug Administration
[17]: Nutrition Label Red Flags: Avoiding Toxic Additives & Chemicals
[18]: The Dirty 30 – Toxic Supplement and Beauty Product Ingredients – Dr. Berg
[19]: What to Avoid with Supplements – ConsumerLab.com
[20]: RECOGNIZE Risk When You See It | U.S. Anti-Doping Agency (USADA)
[21]: 10 Nutrition Label Ingredients That Are Major Red Flags for Your Health
[22]: What Are Adverse Health Effects of PCB Exposure?
[23]: Public Health Statement for PCBs – Centers for Disease Control and …
[24]: Polychlorinated Biphenyls (PCBs) Toxicity: Clinical Assessment …
[25]: Associations between heavy metal exposure and vascular age: a large …
[26]: Office of Food Chemical Safety, Dietary Supplements & Innovation
[27]: Dietary Supplements | FDA – U.S. Food and Drug Administration
[28]: Food/Dietary Supplement Guidance and Regulatory Information
[29]: Navigating FDA Regulations for Dietary Supplements
[30]: Stronger Federal Oversight of Dietary Supplements Will Protect …
[31]: FDA needs stronger rules to ensure the safety of dietary supplements …
[32]: Fact Sheet: Dietary Supplements as Regulated Products
[33]: Third-Party Supplements: Guide to Quality, Certifications, and …
[34]: Why is Third-Party Certification Important for Dietary Supplements? – opss
[35]: Third-Party Certifications: Recognizing Safe Supplements
[36]: The Importance of NSF/ANSI 173 Certification for Dietary… | NSF
[37]: Understanding Supplement Safety for Athletes: Why Third-Party Testing …
[38]: 3rd Party Supplement Testing & Certification | Fullscript
Are your products save?
Download ALVA to check if the products you use regularly are free from dangerous chemicals.

